Understanding Liquids: The Fluid State of Matter and Its Unique Properties
Liquids are essential to life and industry, bridging the gap between rigid solids and chaotic gases. Their unique ability to flow while maintaining volume makes them indispensable. Understanding properties like viscosity and surface tension helps us grasp the fundamental physics of the natural world.
The Science of Liquids: Properties, Examples, and Importance
Matter makes up everything around us, but it doesn't always behave the same way. While solids are rigid and gases float freely, liquids occupy a unique middle ground. They are fundamental to life on Earth—from the water we drink to the blood flowing in our veins.
This guide breaks down exactly what a liquid is, how it behaves, and why it matters in the study of physics and chemistry.
What is a Liquid?
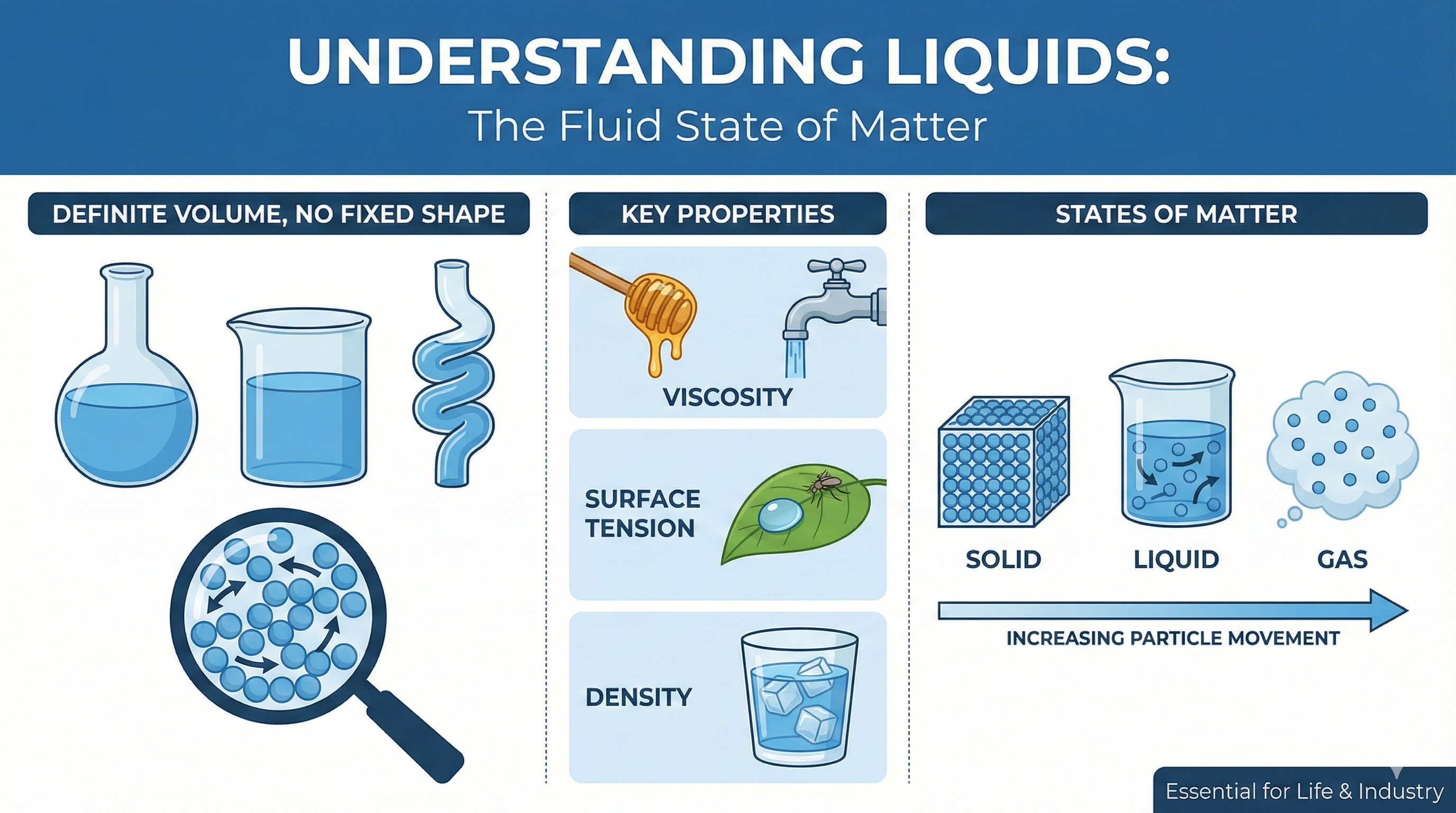
A liquid is one of the distinct states of matter. Unlike a solid, which holds its shape, or a gas, which expands to fill any space, a liquid has a definite volume but no fixed shape.
If you pour water from a pitcher into a cup, the amount of water (volume) stays the same, but the water takes the shape of the cup. This ability to flow is what makes liquids so versatile.
Key Characteristics of Liquids
* Definite Volume: They do not expand to fill a container like gases do.
* No Fixed Shape: They take the shape of whatever holds them.
* Incompressibility: It is very difficult to compress a liquid into a smaller space.
* Fluidity: The particles can slide past one another, allowing movement.
Important Properties of Liquids
To understand how liquids work, we need to look at the forces acting between their molecules. Here are the three most important concepts.
1. Viscosity: The Resistance to Flow
Have you ever noticed that honey pours much slower than water? This is due to viscosity.
* High Viscosity: Fluids that are thick and flow slowly (e.g., honey, syrup, motor oil).
* Low Viscosity: Fluids that flow quickly and easily (e.g., water, alcohol).
Viscosity measures how much internal friction exists within the liquid. The stronger the attraction between molecules, the higher the viscosity.
2. Surface Tension
Surface tension is the reason water droplets form round beads rather than spreading out flat immediately. It acts like an elastic "skin" on the surface of a liquid.
This property allows small insects, like water striders, to walk on water without sinking. It occurs because water molecules at the surface are pulled tightly together by the molecules below them.
3. Density
Density refers to how tightly packed the particles are within the liquid. While most solids are denser than their liquid forms, water is a famous exception—ice is actually less dense than liquid water, which is why ice cubes float.
Liquid vs. Solid vs. Gas
How does a liquid compare to the other primary states of matter?
* Solids: Have a fixed shape and fixed volume. Particles vibrate in place and cannot move freely. They are not compressible.
* Liquids: Take the shape of their container but keep a fixed volume. Particles are close but can slide past each other. They have negligible compressibility.
* Gases: Expand to fill their container and have a changeable volume. Particles move freely and quickly. They are highly compressible.
Why Are Liquids Important?
Liquids are not just a concept in a textbook; they are vital for existence and technology.
* Biological Life: The human body is approximately 60% water. Blood is a liquid tissue that transports oxygen and nutrients.
* Industrial Use: Liquids are used as coolants, lubricants (oil), and solvents in manufacturing.
* Earth's Climate: The oceans (liquid water) regulate the planet's temperature and drive weather patterns.
Supporting Content: Did You Know?
> The Glass Debate: Some people believe old glass is a "supercooled liquid" because old windows look thicker at the bottom. However, this is a myth. Glass is an amorphous solid. It does not flow over time; the unevenness is usually due to how the glass was originally manufactured.
>
Frequently Asked Questions (FAQs)
1. What is the simple definition of a liquid?
A liquid is a state of matter that has a fixed volume but changes its shape to fit its container. The particles are close together but can move around.
2. Can liquids be compressed?
Generally, no. Liquids are considered incompressible for most practical purposes. Unlike gases, you cannot easily force a liquid into a smaller volume because the particles are already touching.
3. Why do liquids flow?
Liquids flow because the intermolecular forces (the bonds holding particles together) are not strong enough to hold the particles in a fixed position, as they do in solids. This allows them to slide over one another.
4. Is fire a liquid?
No. Fire is typically considered a plasma, which is a different state of matter consisting of ionized gas. It is not a liquid.
5. What is the most common liquid on Earth?
Water is the most abundant liquid on Earth, covering about 71% of the planet's surface.